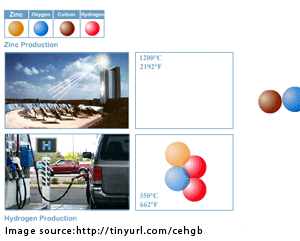
 The Weizmann Institute has released a new study detailing a process to create hydrogen indirectly from zinc-oxide. Basically, the process works by using concentrated solar energy to heat zinc-oxide to a high temperature, thereby transforming it into a gas and resulting in the separation of zinc from oxygen. The zinc later cools and condenses into a powder. Add some water at about 662°F, and the zinc reacts with the water to form zinc-oxide and hydrogen. Now you've got hydrogen and, as a nice little bonus, more zinc oxide with which to start the process anew.
The Weizmann Institute has released a new study detailing a process to create hydrogen indirectly from zinc-oxide. Basically, the process works by using concentrated solar energy to heat zinc-oxide to a high temperature, thereby transforming it into a gas and resulting in the separation of zinc from oxygen. The zinc later cools and condenses into a powder. Add some water at about 662°F, and the zinc reacts with the water to form zinc-oxide and hydrogen. Now you've got hydrogen and, as a nice little bonus, more zinc oxide with which to start the process anew.This article explains the process pretty well, as does this article. These first two articles claim the process "generates no pollution," yet this physorg article states (along with a nice animation):
At a heat of above 1200°C (2192F) the ZnO breaks down into Zn and oxygen which in turn recombines with the carbon to create CO as a minor by-product.This article (which is copied-and-pasted from a Nature article that is only available to premium members, one of which I am not) sheds some light on the carbon-monoxide thing. The process uses coal as a sort of catalyst to reduce the temperature at which zinc-oxide splits into zinc and oxygen, allowing the focused solar energy to provide sufficient heat. The physorg article says, "For the future, the team sees the possibility of replacing the coal completely with biomass thus making the entire process completely pollution free."
But that's not true, either. Just because you're getting the carbon from a "natural" source doesn't mean CO or CO2 won't be produced during the reaction. So there'd still be pollution. The bottom line:
The zinc-forming reaction also releases carbon monoxide from the charcoal, which eventually converts to the greenhouse gas carbon dioxide in the atmosphere. In a full-scale industrial process, the carbon monoxide could be harnessed to help produce even more hydrogen from water. But this too would produce carbon dioxide. For now the process produces as much carbon dioxide as extracting the same amount of hydrogen from natural gas, Epstein says.So, it's not a perfect solution, but that's probably a good thing, because it means it's probably realistic and perhaps commercializable. Is that a word?
Oh yeah, one more thing. The organization that produced this research is the Weizmann Institute, not the Weitzman Institute, as it's referred to in the physorg article.
UPDATE: For comments, see the original blog post here.

No comments:
Post a Comment